Neusignal Therapeutics has completed non-clinical studies of NTX-083, a novel drug candidate for Alzheimer’s Disease (AD) based on a novel Mechanism of Action (MoA), and is now conducting clinical trials.
NTX-083 was discovered by Dr. Shigeki Moriguchi, Associate Professor at Tohoku University and our founding scientist. With support from the Japan Agency for Medical Research and Development (AMED) through the Translational Research Network Program and Interdisciplinary and Emerging Brain Research Program (iBrain/MINDS), we secured non-clinical Proof-of-Concept (POC) and completed two international patent applications filed by Tohoku University. In 2018, Dr. Moriguchi’s team reported the cognitive function improvement effects of memantine, an existing AD treatment, through a novel mechanism of action (Moriguchi S et al., Molecular Psychiatry 2018). Building on this groundbreaking report, we identified NTX-083 through structural optimization and structure-activity relationship analysis of newly synthesized compounds. To prepare NTX-083 for practical application, we conducted and completed GLP toxicology studies for clinical trials of this novel MoA-based dementia therapy. In April 2022, Neusignal Therapeutics, Inc. was founded in Nihonbashi, Chuo-ku, Tokyo. Our company operates based on the patents transferred from Tohoku University.
Studies using AD model mice and other non-clinical research show that NTX-083 improves core symptoms (cognitive function improvement) and peripheral symptoms (psychiatric function improvement), while also modifying the disease. Being orally administrable, it is expected to significantly improve patient access in clinical settings. Additionally, safety studies in various animal models indicate that NTX-083 has high safety profile, among other advantages.
We aim to create a First-in-Class drug that is the world’s first to improve both cognitive and peripheral symptoms while also modifying the disease. With the launch of NTX-083, we strive to offer a new treatment option for dementia to patients and their families.
For over 20 years, only four drugs targeting cognitive function of Alzheimer’s disease were approved.
Recently, disease-modifying drugs have gained approval and drawn attention. We aim to go further, to develop the next generation of treatments for Alzheimer’s disease.
Emergence of drugs like donepezil and memantine, which improve cognitive function to a certain extent
Emergence of drugs like lecanemab and donanemab, which slow the progression of dementia

• Cognitive function improvement
• Peripheral symptom improvement
• Suppression of disease progression

Through the R&D of NTX-083, we have gathered extensive scientific evidence on AD. Using these insights, we are broadening our pipeline beyond NTX-083 by developing backup compounds and exploring new therapeutic targets.
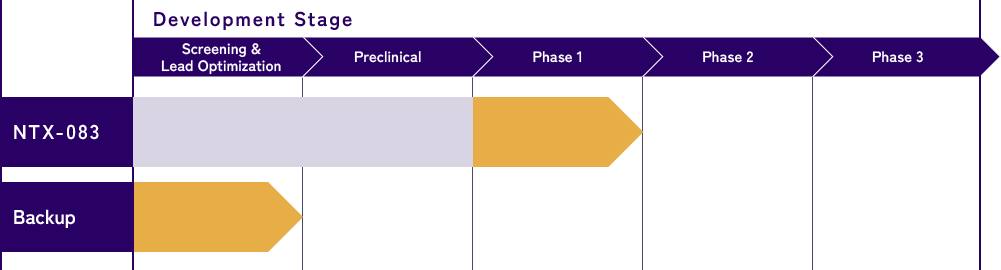
Leveraging the insights from NTX-083’s mechanistic analysis, we are exploring a new targets or in-licensing synergistic programs.